25 Grams Of Hcl To Moles
Webtuts
May 11, 2025 · 4 min read
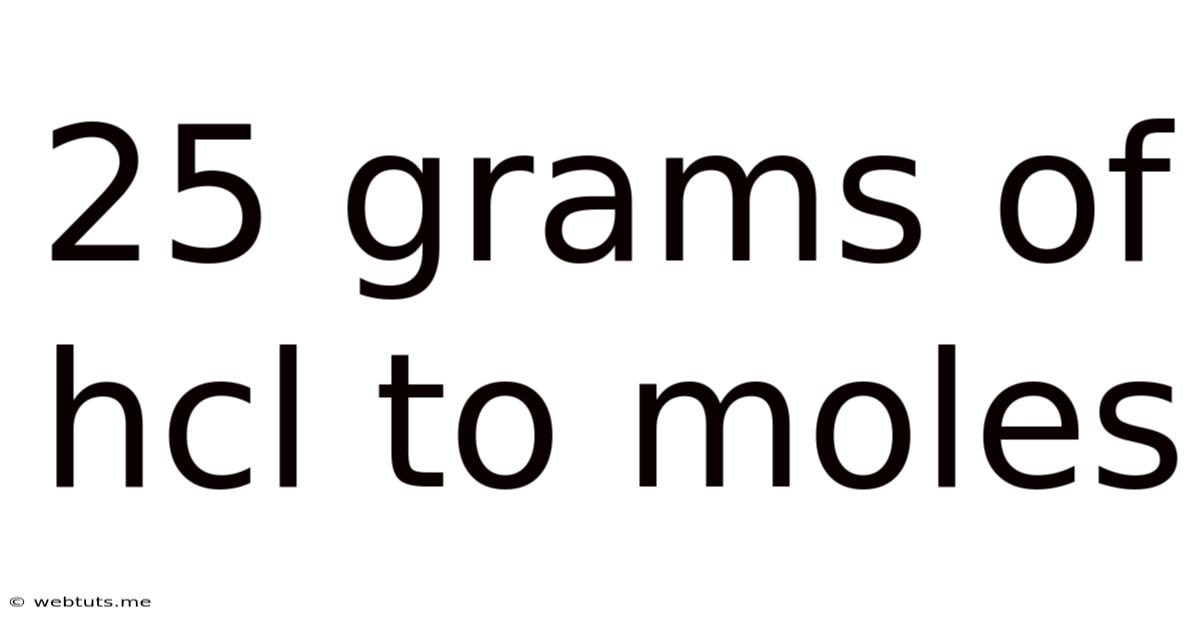
Table of Contents
Converting 25 Grams of HCl to Moles: A Comprehensive Guide
Understanding the conversion of grams to moles is fundamental in chemistry. This process, known as molar mass conversion, is crucial for stoichiometric calculations, determining reaction yields, and preparing solutions of specific concentrations. This in-depth guide will walk you through the steps involved in converting 25 grams of hydrochloric acid (HCl) to moles, explaining the underlying concepts and providing practical examples.
Understanding Moles and Molar Mass
Before diving into the conversion, let's clarify the core concepts:
-
Mole (mol): A mole is a fundamental unit in chemistry, representing a specific number of entities, such as atoms, molecules, or ions. This number, known as Avogadro's number, is approximately 6.022 x 10<sup>23</sup>. Essentially, a mole is a convenient way to count extremely large numbers of tiny particles.
-
Molar Mass (g/mol): Molar mass represents the mass of one mole of a substance. It's expressed in grams per mole (g/mol). The molar mass of a compound is the sum of the atomic masses of all atoms in its chemical formula.
Calculating the Molar Mass of HCl
To convert grams of HCl to moles, we first need to determine the molar mass of HCl. This involves adding the atomic masses of hydrogen (H) and chlorine (Cl).
- Atomic mass of Hydrogen (H): Approximately 1.008 g/mol
- Atomic mass of Chlorine (Cl): Approximately 35.45 g/mol
Therefore, the molar mass of HCl is:
1.008 g/mol (H) + 35.45 g/mol (Cl) = 36.458 g/mol
Converting 25 Grams of HCl to Moles
Now that we know the molar mass of HCl, we can perform the conversion. The formula for converting grams to moles is:
Moles = Mass (grams) / Molar Mass (g/mol)
Let's plug in the values:
Moles = 25 g / 36.458 g/mol
Moles ≈ 0.686 moles
Therefore, 25 grams of HCl is approximately equal to 0.686 moles.
Practical Applications and Significance
The ability to convert grams to moles is essential in various chemical applications:
-
Stoichiometric Calculations: In balanced chemical equations, the coefficients represent the molar ratios of reactants and products. Converting grams to moles allows you to determine the amount of reactants needed or products formed in a chemical reaction. For example, if you're reacting HCl with a metal to produce hydrogen gas, knowing the moles of HCl will help you calculate the amount of hydrogen gas produced.
-
Solution Preparation: Many chemical reactions and experiments require solutions of specific concentrations, typically expressed in molarity (moles per liter). Converting grams to moles is necessary to prepare solutions with the desired concentration.
-
Titration Calculations: Titration is a common analytical technique used to determine the concentration of an unknown solution. The calculations involved in titration rely heavily on converting grams to moles to determine the concentration of the unknown substance.
-
Determining Empirical and Molecular Formulas: The conversion of grams to moles is fundamental in determining the empirical and molecular formulas of compounds. By knowing the mass of each element in a compound, you can convert to moles and then determine the simplest whole-number ratio of atoms.
Potential Sources of Error and Precision
While the calculation is straightforward, several factors can introduce errors:
-
Impurities in the HCl: If the HCl sample contains impurities, the actual amount of HCl might be less than 25 grams, leading to an inaccurate mole calculation. Using a high-purity sample minimizes this error.
-
Rounding Errors: Rounding off the atomic masses and the final answer can introduce small errors. Using more significant figures during the calculation reduces these errors.
-
Measurement Errors: Inaccurate weighing of the HCl sample will directly impact the accuracy of the mole calculation. Using a precise balance is crucial.
Advanced Applications and Related Concepts
The concept of molar mass conversion extends beyond simple compounds like HCl. It's applicable to more complex molecules, mixtures, and even substances with varying isotopic compositions. Understanding these complexities requires a deeper dive into:
-
Isotopic Abundance: Elements exist as isotopes with different atomic masses. The molar mass used in calculations is the weighted average of the masses of all isotopes.
-
Hydrates: Many compounds exist as hydrates, which contain water molecules bound to the main compound. Calculating the molar mass of a hydrate requires considering the mass of the water molecules.
-
Mixtures: The molar mass of a mixture is a weighted average of the molar masses of its components, based on their respective mass fractions.
-
Percent Composition: Knowing the percent composition of a compound allows for the calculation of its empirical and molecular formulas, leveraging the conversion between grams and moles.
Conclusion
Converting 25 grams of HCl to moles is a fundamental chemical calculation with broad applications. Mastering this conversion is essential for success in various chemical endeavors. Remember to always use accurate measurements, consider potential sources of error, and understand the underlying concepts to ensure accurate and reliable results. By consistently applying these principles, you will enhance your ability to confidently perform and interpret chemical calculations, fostering a strong foundation in your understanding of chemistry. Understanding the nuances of molar mass and its role in different chemical contexts will empower you to tackle more complex problems and deepen your overall comprehension of chemistry principles.
Latest Posts
Latest Posts
-
How Do I Figure Out My Raise Percentage
May 12, 2025
-
How Many Days Left In October 2024
May 12, 2025
-
How Many Hours Is 24 Days
May 12, 2025
-
How Many Tablespoons Makes 2 3 Of A Cup
May 12, 2025
-
How Many Cups In 6 Oz Of Water
May 12, 2025
Related Post
Thank you for visiting our website which covers about 25 Grams Of Hcl To Moles . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.