How Many Mg In 1.5 Ml
Webtuts
Apr 04, 2025 · 5 min read
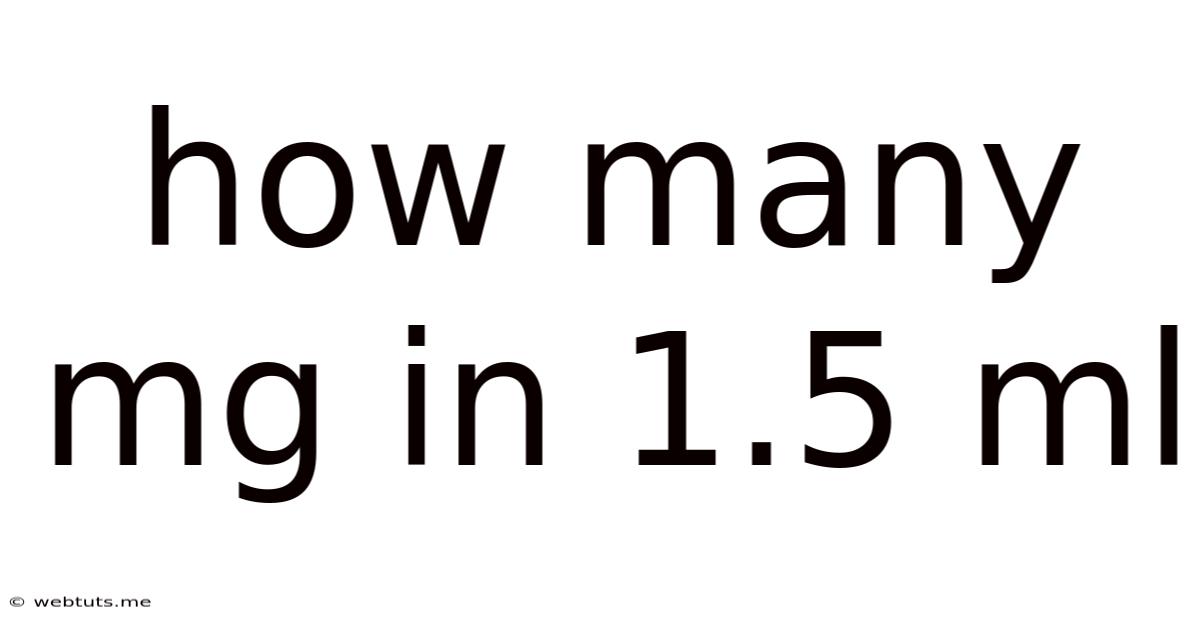
Table of Contents
How Many mg in 1.5 ml? Understanding Concentration and Conversions
Determining how many milligrams (mg) are in 1.5 milliliters (ml) isn't a straightforward answer. It entirely depends on the concentration of the substance in question. Concentration refers to the amount of a substance (solute) dissolved in a specific amount of liquid (solvent). This is usually expressed as milligrams per milliliter (mg/ml), milligrams per liter (mg/L), or even as a percentage (%). Let's delve deeper into understanding these concepts and how to perform the necessary calculations.
Understanding Concentration: The Key to Conversion
Before we can answer "how many mg in 1.5 ml?", we need to know the concentration of the solution. This crucial piece of information tells us how many milligrams of the solute are present in each milliliter of the solution.
For example:
- A solution with a concentration of 100 mg/ml means that there are 100 milligrams of the solute in every 1 milliliter of the solution.
- A solution with a concentration of 250 mg/L means that there are 250 milligrams of the solute in every 1 liter (1000 ml) of the solution. To use this in our calculation, we'd need to convert it to mg/ml.
Calculating mg from ml: Different Scenarios
Let's explore different scenarios and the calculations involved:
Scenario 1: Concentration given in mg/ml
This is the simplest scenario. If we know the concentration in mg/ml, we can directly calculate the amount in 1.5 ml.
Example: A solution has a concentration of 50 mg/ml. How many mg are in 1.5 ml?
Calculation:
50 mg/ml * 1.5 ml = 75 mg
Therefore, there are 75 mg in 1.5 ml of this solution.
Scenario 2: Concentration given in mg/L
If the concentration is given in mg/L, we must first convert it to mg/ml before performing the calculation. Remember that 1 liter (L) equals 1000 milliliters (ml).
Example: A solution has a concentration of 250 mg/L. How many mg are in 1.5 ml?
Conversion to mg/ml:
250 mg/L * (1 L / 1000 ml) = 0.25 mg/ml
Calculation:
0.25 mg/ml * 1.5 ml = 0.375 mg
Therefore, there are 0.375 mg in 1.5 ml of this solution.
Scenario 3: Concentration given as a percentage (%)
A percentage concentration indicates the amount of solute per 100 ml or 100 g of solution. This requires conversion to mg/ml before the calculation. We need to know whether the percentage is weight/volume (%) w/v or weight/weight (%) w/w.
- Weight/Volume (% w/v): This means grams of solute per 100 ml of solution.
Example: A solution has a concentration of 5% w/v. How many mg are in 1.5 ml?
Conversion to mg/ml:
5 g/100 ml * (1000 mg/1 g) = 50 mg/ml
Calculation:
50 mg/ml * 1.5 ml = 75 mg
Therefore, there are 75 mg in 1.5 ml of this 5% w/v solution.
- Weight/Weight (% w/w): This means grams of solute per 100 g of solution. This is more complex and requires knowing the density of the solution to accurately convert to mg/ml. Density is typically expressed in g/ml.
Scenario 4: Unknown Concentration – The Importance of Labeling
Crucially, if you don't know the concentration, you cannot determine the amount in mg. This is why accurate labeling is so important, especially in pharmaceutical, chemical, and food settings. Never attempt to estimate or guess the concentration.
Practical Applications and Safety Considerations
Understanding how to convert between mg and ml is crucial in many fields, including:
- Medicine: Calculating medication dosages.
- Chemistry: Preparing solutions for experiments.
- Food science: Determining the amount of additives or nutrients in a food product.
Safety is paramount: Always double-check calculations and refer to official instructions or consult a professional when handling substances with unknown concentrations. Incorrect calculations can have serious consequences in medication or chemical handling.
Common Mistakes to Avoid
- Ignoring units: Always pay close attention to the units (mg/ml, mg/L, %) and perform necessary unit conversions.
- Incorrect conversions: Make sure you are correctly converting between units like liters and milliliters, grams and milligrams.
- Assuming concentration: Never assume the concentration; always refer to the label or provided information.
Advanced Considerations: Density and Specific Gravity
For highly precise calculations, particularly when working with solutions that are not water-based, the density of the solution must be considered. Density, expressed in g/ml or g/cm³, is the mass per unit volume. If you know the density and the concentration in g/ml or %, you can use these values to calculate the mass (in mg) present in 1.5 ml.
Beyond Simple Conversions: Molarity and Molality
For more advanced chemical applications, concentration might be expressed in molarity (moles/liter) or molality (moles/kilogram). These expressions require a deeper understanding of chemical principles and stoichiometry to convert them to mg/ml.
Conclusion: Accuracy and Precision in Calculations
Determining how many mg are in 1.5 ml hinges entirely on the concentration of the substance. The examples and scenarios outlined above provide a framework for performing these conversions accurately. Always prioritize accuracy and precision, and never hesitate to seek professional guidance when dealing with unfamiliar substances or complex calculations. Remember that safety should always be the top priority when working with any substance, particularly medications or chemicals. Precise calculations are vital to prevent errors and ensure the safe and effective use of various solutions.
Latest Posts
Latest Posts
-
How Many Days Until December 13th 2024
May 10, 2025
-
How Many Seconds In 15 Min
May 10, 2025
-
16 Oz Of Flour To Cups
May 10, 2025
-
How Many Quarts Are In 30 Gallons
May 10, 2025
-
How Many Days Till November 23 2024
May 10, 2025
Related Post
Thank you for visiting our website which covers about How Many Mg In 1.5 Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.