How Many Ml Is 6 Mg
Webtuts
May 13, 2025 · 4 min read
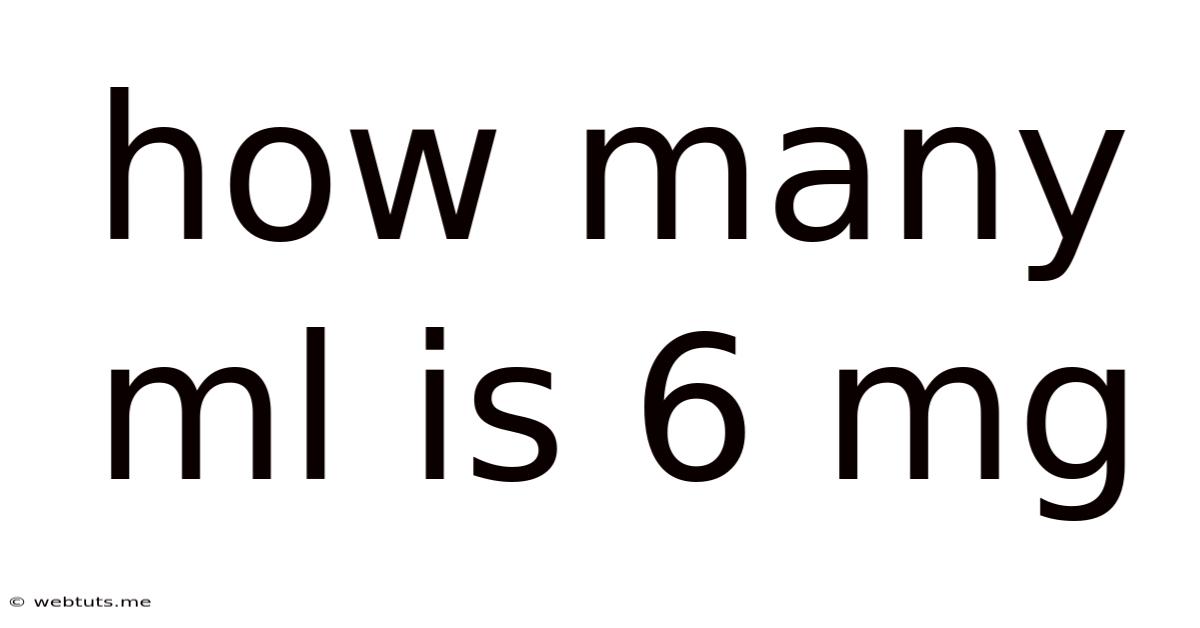
Table of Contents
How Many mL is 6 mg? Understanding Concentration and Dosage
The question "How many mL is 6 mg?" cannot be answered without knowing the concentration of the solution. Milliliters (mL) represent volume, while milligrams (mg) represent mass or weight. To convert between the two, you need a crucial piece of information: the concentration of the substance in the solution, typically expressed as mg/mL (milligrams per milliliter) or a similar unit. This article will explain the concepts involved, provide examples, and highlight the critical importance of accuracy in medication and other applications.
Understanding Concentration
Concentration describes the amount of solute (the substance dissolved) present in a given amount of solvent (the substance doing the dissolving), usually expressed as a ratio or percentage. Common units include:
- mg/mL (milligrams per milliliter): This indicates the number of milligrams of solute present in one milliliter of solution. This is a very common unit in pharmaceutical contexts.
- g/L (grams per liter): This is another common unit, often used in larger-scale applications.
- % (w/v) (percent weight per volume): This represents the grams of solute per 100 mL of solution.
The concentration is crucial for converting between mass (mg) and volume (mL). Without it, the conversion is impossible.
The Calculation: mg to mL Conversion
Once you know the concentration, the conversion is straightforward:
mL = mg / (mg/mL)
Let's break this down:
- mg: This is the mass of the substance you're interested in (in this case, 6 mg).
- mg/mL: This is the concentration of the solution, given as milligrams of solute per milliliter of solution.
Example 1: A simple conversion
Let's say you have a solution with a concentration of 2 mg/mL. How many mL would contain 6 mg of the solute?
mL = 6 mg / (2 mg/mL) = 3 mL
Therefore, 3 mL of this 2 mg/mL solution would contain 6 mg of the solute.
Example 2: A more complex scenario
Imagine you have a medication with a concentration of 250 mg/5 mL. How many mL would contain 6 mg of the medication?
First, we need to calculate the concentration in mg/mL:
Concentration = 250 mg / 5 mL = 50 mg/mL
Now we can use the formula:
mL = 6 mg / (50 mg/mL) = 0.12 mL
Therefore, 0.12 mL of this medication would contain 6 mg.
The Importance of Accuracy
Accurate conversion between mg and mL is extremely important in many fields, especially in:
-
Pharmaceuticals: Incorrect dosages can have serious, even life-threatening consequences. Healthcare professionals must carefully calculate dosages based on the concentration of medications. Always follow the instructions provided by your doctor or pharmacist. Never attempt to calculate dosages yourself without proper training.
-
Chemistry and Laboratory Work: Accurate measurements are essential for experiments and analysis. Incorrect dilutions or concentrations can lead to inaccurate results and compromised experiments.
-
Food and Beverage Industry: Maintaining consistent concentrations is crucial for quality control and ensuring products meet the required specifications.
-
Environmental Science: Accurate measurements of pollutants and other substances are necessary for monitoring and remediation efforts.
Potential Sources of Error
Several factors can contribute to errors in mg to mL conversions:
-
Incorrect Concentration: The most common source of error is using an incorrect concentration value. Always double-check the label or documentation to ensure you're using the correct concentration.
-
Measurement Errors: Inaccurate measurements of either mass or volume can lead to significant errors in the final calculation. Use accurate measuring devices and techniques.
-
Rounding Errors: Rounding off numbers too early in the calculation can lead to accumulated errors. It's best to use the full precision of your calculator throughout the calculation.
-
Unit Inconsistencies: Ensure that all units are consistent throughout the calculation. Mixing units (e.g., using grams and milligrams) will lead to incorrect results.
Beyond Simple Conversions: Diluting Solutions
Often, you might need to dilute a more concentrated solution to achieve a specific concentration. This requires a different type of calculation. A common method uses the formula:
C1V1 = C2V2
Where:
- C1 = initial concentration
- V1 = initial volume
- C2 = desired concentration
- V2 = desired volume
Example: Diluting a Solution
You have a 100 mg/mL solution and need to prepare 10 mL of a 20 mg/mL solution. What volume of the 100 mg/mL solution do you need?
C1V1 = C2V2
(100 mg/mL) * V1 = (20 mg/mL) * 10 mL
V1 = (20 mg/mL * 10 mL) / (100 mg/mL) = 2 mL
You would need 2 mL of the 100 mg/mL solution and dilute it to a final volume of 10 mL to obtain a 20 mg/mL solution. Remember to add the solvent (e.g., water) carefully to reach the desired volume.
Conclusion: The Importance of Precision and Understanding
Converting between milligrams and milliliters requires a precise understanding of concentration. This conversion is critical in many applications, particularly those involving medication and scientific work. Always double-check your calculations, use accurate measuring devices, and understand the potential sources of error. If you are working with medications, always consult with a healthcare professional for dosage instructions. Never attempt to calculate dosages on your own unless you have proper training. Precision and accuracy are paramount to ensure safety and reliable results. Understanding the underlying principles discussed in this article will greatly enhance your ability to perform these conversions correctly and confidently.
Latest Posts
Latest Posts
-
How Many Quarts Is 27 Gallons
May 13, 2025
-
How Many Mg In 2 5 Ml
May 13, 2025
-
96 Inches Equals How Many Feet
May 13, 2025
-
How Many More Days Till September 10
May 13, 2025
-
How Many Minutes Is 160 Seconds
May 13, 2025
Related Post
Thank you for visiting our website which covers about How Many Ml Is 6 Mg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.